Give at least 2 objects that can be used to perform following activities 1. Sul Get the answers you need now.
Periodic Trends In Electronegativity Ck 12 Foundation
90 - Melting point.

. H 2 O molecules have a larger diameter. 05062016 Question 00241032 Subject Chemistry Topic General Chemistry Tutorials. The element in period 4 that has the greatest electronegativity is Fluorine.
It does take electrons from the atoms of. Waec-2020 ssce-exam-2020 waec-chemistry-2020 chemistry. And those three elements are fluorine oxygen and nitrogen.
Water and carbon dioxide exist in different states because. 18K views Share Follow. A Si B Br C Cl D P Medium Solution Verified by Toppr Correct option is C Cl has the highest electronegativity due to its high electron affinity value.
So among the given elements the electronegativity of Phosphorous is highest. 200 Posted By. What are the 3 most electronegative elements.
F A sample of nitrogen gas has a volume of 224 L at 200 C. Chlorine has the greatest electronegativity because out of all the choices it lies farthest to the right and top of periodic table. What is the final volume in liters of nitrogen gas.
Which of these elements has the greatest electronegativity. Which of the following elements has the greatest electronegativitya. Which of the following pairs of elements has the greatest difference in electronegativity.
Which of the following elements has the greatest electronegativity Offered Price. 04062016 1216 AM Due on. At standard pressure and room temperature water H 2 O is a liquid and carbon dioxide CO 2 is a gas.
Chlorine has a great attraction for electrons in a chemical bond because it needs only one more electron to complete a stable octet formation. Thus c is the right option. What 3 elements have the highest electronegativity.
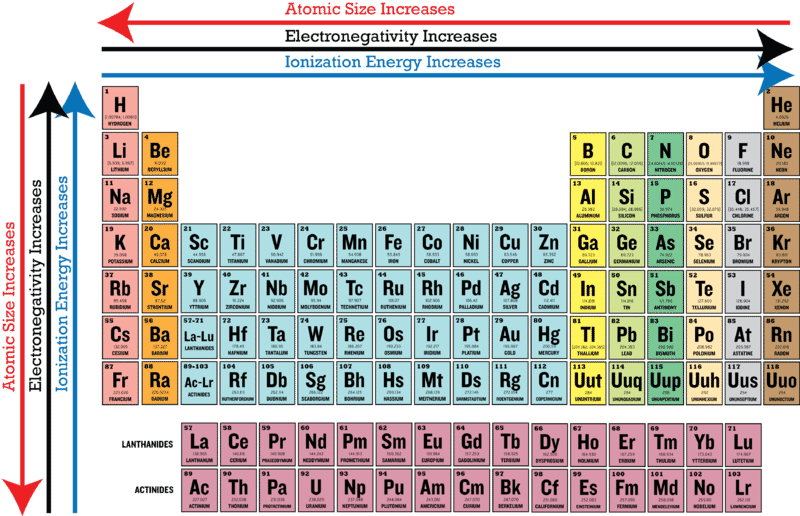
Mg bS cSr d. Arrange the following elements in order of increasing electronegativity. Electronegativity will increase from left to right in the period.
The gas is heated to 146 C at consant pressure. Of the following elements which one has the highest electronegativity. And those three elements are fluorine oxygen and nitrogen.
Solve any question of Classification Of Elements And Periodicity In Properties with- Patterns of problems. The electronegativity of nitrogen itself although lower than that of oxygen is substantially higher than that of any of the other elements other than Fluorine which is the most electronegative out of all the elements. Fluorine is the most electronegative element on the peri View the full answer.
And in fact the reason why theyre capable is hydrogen bonds is because these three are the most electronegative elements. Fluorine F has the highest electronegativity 40. BS Silicon 18 ang electronegativity.
Therefore it has a high electronegativity. Which element has the greatest electronegativity N P. Rb 2 See answers Advertisement Advertisement Daniela448 Daniela448 Answer.
Which of the following pairs of elements has the greate. 119 rows ELEMENT ELECTRONEGATIVITY. Arrange the following elements in order of increasing electronegativity.
CO 2 molecules have a greater mass. Which of the following elements has the greatest electronegativity Select one. It does take electrons from the atoms of other elements.
Fluorine F has the highest electronegativity 40. The bonds are stronger is CO 2 molecules. Which is more electronegative N or O.
And in fact the reason why theyre capable is hydrogen bonds is because these three are the most electronegative elements. Along the period electronegativity increases and down the group it decreases. They have the same number of valence electrons so they react similarly.
118 rows - Electronegativity.

The Parts Of The Periodic Table

The Parts Of The Periodic Table

How Can We Know That Which Element Is More Electronegative Than Other I Ve Never Come Across Any Such Formula Or Trick For This Except Learning It By Practice Please Tell If You
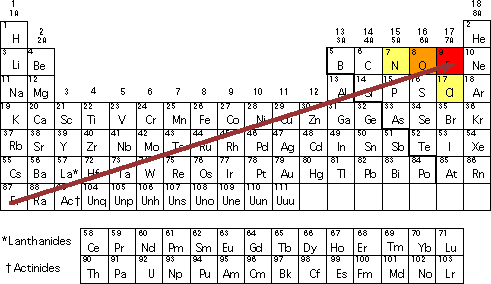
0 Comments